Christopher Athmer PE & Brent Huntsman PG
Terran Corporation
Remediation of clay and silt laden soils continues to provide the biggest challenge in the soil remediation arena. The limited pore water and contaminant movement in tight soils poses problems for traditional soil cleansing processes. Processes like pump-and-treat, soil vapor extraction, in-situ oxidation and even thermal processes are usually inadequate for removing contaminants from fine grain soils. These processes generally rely on diffusion of the chemicals through clays and silts into regions of higher permeability where they are transported to capture or treatment wells. Diffusion through soil can be very slow. The operation time for these processes are often measured in years to decades. Even systems operating in more permeable soils have difficulty reaching the clean up targets due to clay boundary layers or clay strata within the treated area.
There is a possible solution: electroosmosis. Simply put, electroosmosis is the movement of water (and whatever is contained in the water) through a porous media by applying a direct current (DC) field. In this case, the porous media is soil. A brief review of the science behind the application of electroosmosis for contaminant remediation is the focus of this paper.
When DC is applied to the soil volume, pore water and most contaminants move from the anode (the positive electrode) to the cathode (the negative electrode). Figure 1 shows graphically the prevailing theory as to what is happening within a soil pore in an electric field.
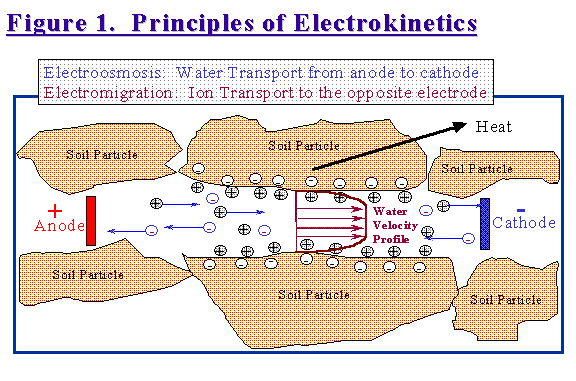
Soil typically has a negative surface charge. To balance this charge, a row of cations such as sodium, calcium and magnesium line up along the soil particle surfaces. There may be other ions in the bulk pore water solution, but these are balanced as far as cations and anions. Common counter-anions in soil water are chloride, sulfate and nitrate. Under the influence of a DC field, the rows of cations on the soil particle surfaces start sliding along towards the cathode by electrical attraction. The movement of this boundary layer of cations drags the bulk soil water with it. Within the bulk soil water, the individual cations also move towards the cathode and the anions move towards the anode. The movements of these individual ions mostly cancel each other out and have no real effect on the bulk water transport. This ion movement however, called electromigration, is very important when treating soils contaminated with metals, nitrates, sulfates or other inorganic compounds.
Electromigration has been used in a limited role of late for the remediation of metal contaminated soils at industrial sites, such as electroplating facilities, with varying degrees of success. One of the problems with electromigration of metals through the soil is the pH control required at the cathodes. When a DC voltage is applied, electrolysis occurs according to the following reactions:
![]()
At the anode, water is oxidized releasing oxygen and creating protons (H+) resulting in an acid front moving towards the cathode. At the cathode, hydrogen is evolved and a base front of hydroxyl ions (OH-) is generated and moves towards the anode. The metal ions migrating towards the cathode will tend to precipitate as metal hydroxides when they reach the base front. This prevents the metals from reaching the cathode where they can be removed or plated out. Specialized electrodes can be utilized to prevent the base or acid generation at the electrodes but tend to be costly and require higher maintenance and operation costs.
Using electroosmosis for organics removal, however, reduces the need for specialized electrodes since most organic contamination is not affected by pH. Trichloroethylene, for instance, will not precipitate or bind to soil any differently at higher pH. The only critical aspect of electrode operations for electroosmosis is that they are hydraulically as well as electrically conductive. The anodes need to stay wet to supply the moisture moving away towards the cathode. If pore water is not replaced, the anode will desiccate and the soil electrical conductance will diminish causing the process to shut down.
With electroosmosis, a higher pH actually increases pore water movement. Acid build-up at the anode, however, slows down the electroosmotic flow due to accumulation of protons on the soil surface. Protons are very small and do not "drag" much water with them as they move toward the cathode. At extremely low pH, reverse flow (towards the anode) has been observed. A simple solution to this problem is the use of recycled flow water. By collecting the high pH water at the cathode, treating it if necessary and putting it back in at the anode, the pH of the anode will remain acceptable. Another technique is to use a sacrificial anode made of steel. Iron will oxidize preferentially over water limiting the generation of H+. These two measures together help maintain a near neutral pH at the anode and result in stable long-term electroosmotic flow rates. The costs of these measures are minimal. LasagnaTM, an electroosmosis based remediation process in use at the Paducah Gaseous Diffusion Plant in Kentucky makes use of these measures. Cathode water flows by gravity back to the anodes for recharge and ¼" steel plates are used as electrodes. Sufficient iron is available to continue operation longer than the expected two-year operation time.
Electroosmosis provides two benefits when properly applied. First, electroosmosis provides uniform pore water movement in most types of soil. Since the boundary layer movement towards the cathode provides the motive force for the bulk pore water, the size of the pore is not important. Unlike hydraulic conductivity, electroosmotic flow rate is NOT sensitive to pore size. Electroosmotic flow rate is primarily a function of applied voltage. The electroosmotic permeability for any soil at 20oC is around 1 x 10-5 cm/sec at 1 volt/cm. The entire soil mass between the electrodes is basically treated equally. This is why electroosmosis is so effective in clayey and heterogeneous soils.
The second major benefit is the electricity applied to the soil directly results in heating of the soil. The soil warming not only increases the mobilization of volatile organics but also increases the electroosmotic permeability by lowering the viscosity of the pore water.
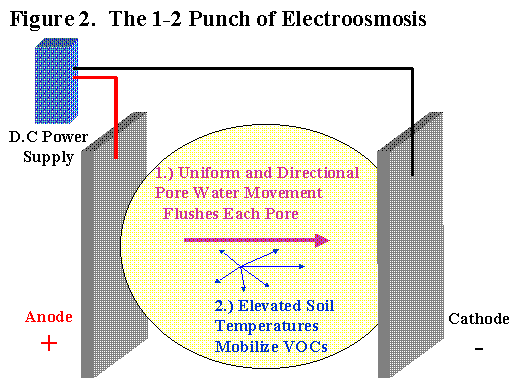
The applied DC voltage and induced current provide resistive heating power to the entire soil mass between the electrodes. The current is a function of the soil electrical conductivity according to Ohm’s law. Soils that are more electrically conductive will draw more current at a given voltage gradient and generate more heat. Highly conductive soils, such as salt water coastal flood plains, are not good candidates for electroosmosis due to the extremely high current draw. Current draw determines the amount of the electricity needed (kilowatt-hours) which directly impacts the operating costs. For most sites, the highest tolerated soil temperature will ultimately determine the current input at steady state. The soil heating aspect alone means electroosmosis should be as effective as other thermal extraction or treatment systems. Direct electrical heating is more cost effective than steam injection or radio frequency (RF) because rectifiers and transformers are less expensive to buy and maintain than portable steam plants or RF generators.
The most exciting results obtained so far show that electroosmosis can be used to effectively treat clay soils highly contaminated with chlorinated solvents. Ho, Athmer Et. Al.1 showed in a field demonstration that trichloroethylene (TCE), even in pure phase form (or DNAPL) is mobilized by electroosmosis. This might be made possible by the severe lowering of the viscosity of TCE to the point where the electroosmotic pressure is great enough to push the TCE through the soil. The soil temperatures can easily reach 80 Deg C in a moderately (electrical) conductive soil.
Electroosmosis systems can be easily adapted to many types of treatment schemes. In-situ and ex-situ systems can be used. In-situ reactive walls are used by the Lasagna system to degrade TCE in the soil. Electroosmosis enhancement of soil vapor extraction systems (SVE) has been demonstrated. SVE systems that have reached the diffusion-limited state can be revitalized by an electroosmosis overlay, which takes advantage of the thermal as well as the clay cleansing benefits. By co-locating the cathodes with the extraction wells and adding a series of anode wells, an inexpensive boost can be made to these systems. Similar enhancement can be given to pump-and-treat systems.
Convective systems like pump-and-treat, vapor extraction, surfactant flushing or in-situ oxidation cannot match the effectiveness of electroosmosis in treating soils with silts or clays present. These systems favor the higher permeability zones. Steam based thermal systems cannot match the uniform soil heating resulting from the DC power input using electroosmosis. Radio Frequency or 6-Phase soil heating may have uniform heating but cannot match the pore water movement. Dynamic underground stripping or steam enhanced vapor extraction are still limited by diffusion in tighter clays. Table 1 shows the advantage of using electroosmosis.
Table 1. Comparison of Treatment Technologies
| Remediation Process | Transport in Soil | Heating Pattern |
| SVE | Preferential/non-uniform | None |
| Pump and Treat | Preferential/non-uniform | None |
| Steam Stripping | Preferential/non-uniform | Non-uniform |
| Surfactant Flushing | Preferential/non-uniform | None |
| In-Situ Chemical Oxidation | Preferential/non-uniform | Non-uniform (low) |
| 6-Phase or RF Soil Heating | None | Uniform |
| Electroosmosis | Uniform | Uniform |
In conclusion, electroosmosis is the most effective tool when treating heterogeneous, silt and clay rich soil. The dual benefits of uniform soil heating and uniform pore water movement make electroosmosis the most thorough soil treatment system available.
1 Ho, Sa V., C.J. Athmer, P.W. Sheridan, B.M. Hughes, R. Orth, D. McKenzie, P.H. Brodsky, A.M. Shapiro, T.M Sivavec, J Salvo, D. Schultz, R. Landis, R. Griffith, and S. Shoemaker, "The Lasagna Technology for In Situ Soil Remediation. 2. Large Field Test", Environmental Science & Technology, Vol 33, No.7, 1999, pp. 1092-1099.
Lasagna is a Trademark of Monsanto, St. Louis, MO.
